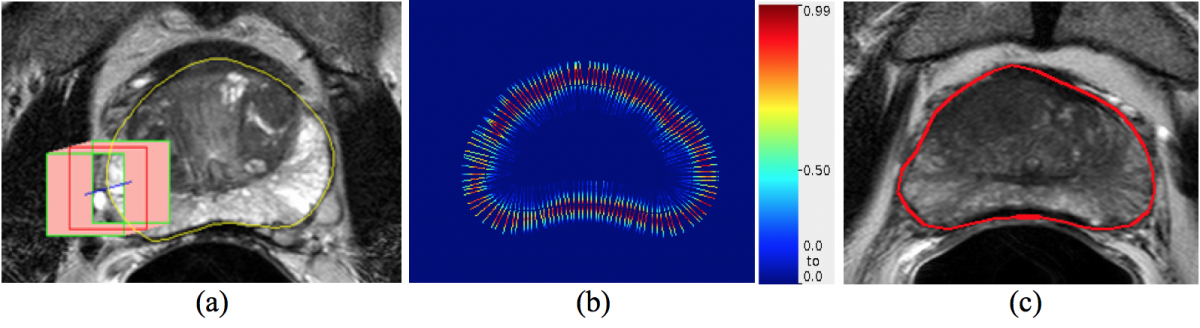
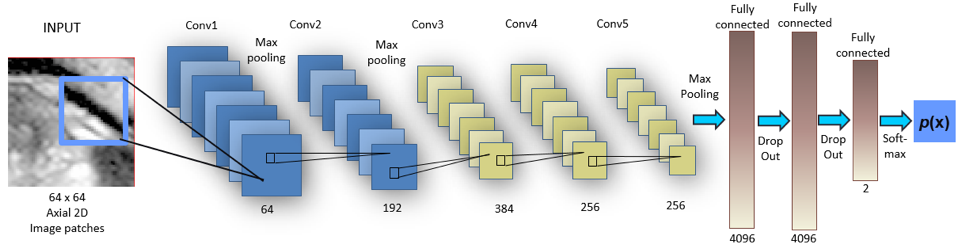
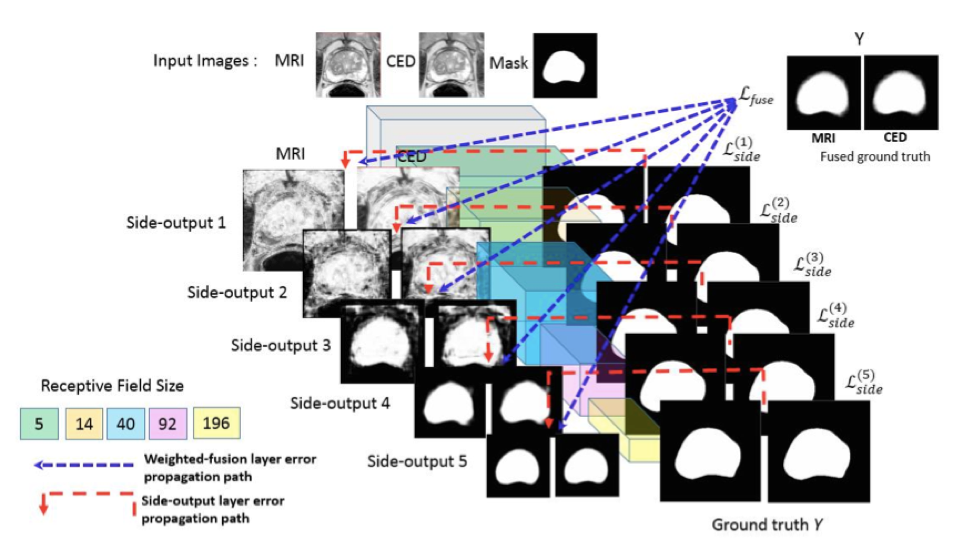
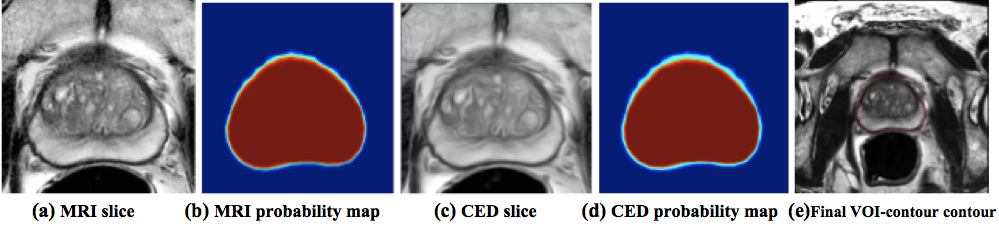
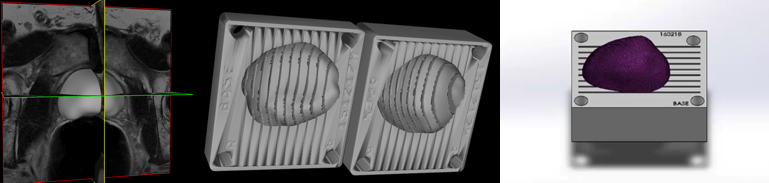
MRI PROSTATE SEGMENTATION
PROJECT COLLABORATORS
NIH, Clinical Center, Imaging Biomarkers and CAD Laboratory:
Ronald M. Summers (MD, PhD, Chief, Director), Holger R. Roth (PhD), Nathan Lay (PhD), Le Lu (PhD)
NCI, Molecular Imaging Program:
Peter Pinto (MD, Chief, Director)
NCI, Center of Cancer Research, Urologic Oncology Branch:
Peter Pinto (MD, Chief, Director)
NIH, Clinical Center, Center of Interventional Oncology:
Brad Wood (MD, Chief, Director)
CIT, OIR, Signal Processing and Instrumentation Section:
Tom Pohida (PhD, Chief)