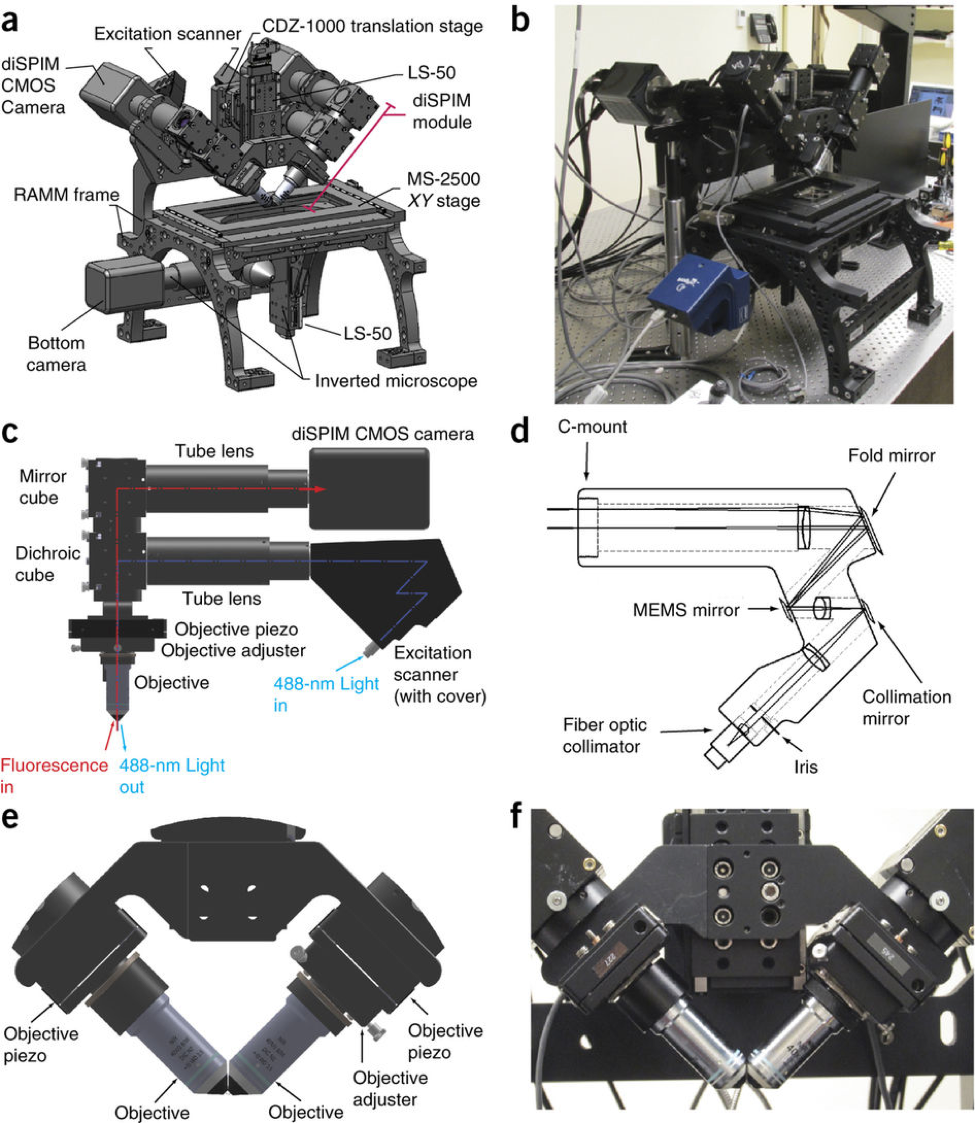
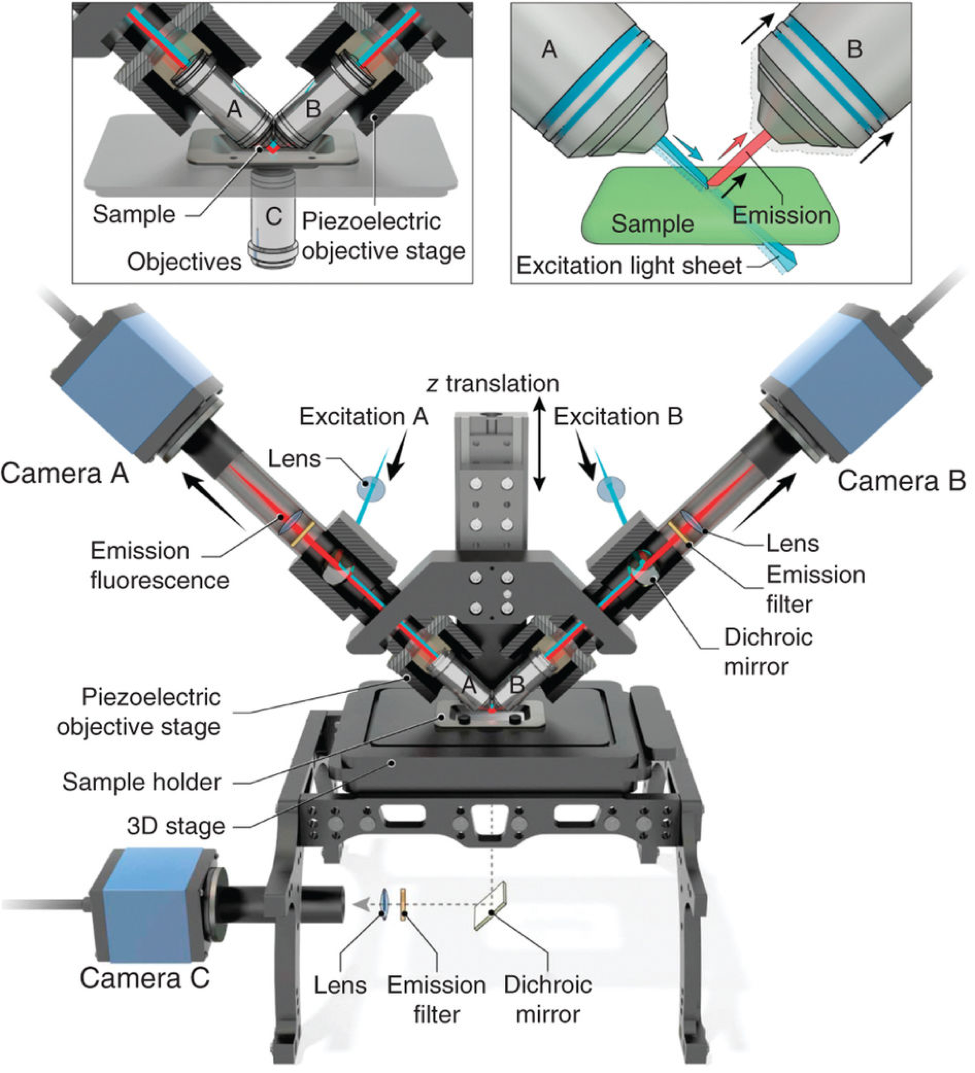
DUAL-VIEW ISPIM IMAGING
PROJECT COLLABORATORS
Section on High Resolution Optical Imaging, NIBIB, NIH:
Hari Shroff, Ph.D, Ryan Patrick Christensen, Ph.D, Yicong Wu, Ph.D, Abhishek Kumar, Ph.D, Panagiotis Chandris, Peter Wawrzusin, Andrew G. York, Peter W. Winter.
National Heart, Lung, and Blood Institute, NIH:
Robert S. Fischer, Clare M. Waterman.
Developmental Biology Program, Sloan-Kettering Institute:
Zhirong Bao, Anthony Santella.
Program in Cellular Neuroscience, Neurodegeneration and Repair, Department of Cell Biology, Yale University School of Medicine:
Daniel A Colón-Ramos.
Applied Scientific Instumentation:
Gary Rondeau.